In the late 1700s, work by Lavoisier and Proust led Dalton to present a theory that all matter is composed of tiny particles called atoms. J.J. Later on in the late 1800s, electrons were discovered in cathode ray tubes which led scientists to think that atoms had a structure and had smaller parts. J.J. Thomson proposed the 'plum pudding' model of the atom (see Figure 1 below).
Figure 1: The 'Plum Pudding' model of the atom. (Source: https://upload.wikimedia.org/wikipedia/commons/thumb/2/26/Plum_pudding_model.svg/2000px-Plum_pudding_model.svg.png)
Another scientist called Ernest Rutherford had spent time working with radioactive materials and the radiation that they produce alongside Paul Viliard and decided to test Thomson's 'plum pudding' model. He hung up a very thin sheet of gold foil, surround it with detectors that could detect alpha particles (positively charged) and from the positions of the scattered alpha particles, he could decipher the structure of the atom. Rutherford found that most of the alpha particles passed straight through the gold foil, a few were scattered slightly deflected and a tiny amount bounced straight back (see Figure 2). He concluded that atoms were mainly empty space with all of the mass and positive charge and mass of the atom was contained in a tiny space at the very centre of the atom (see Figure 3). This space was called the nucleus.
Figure 2: Rutherford's gold foil experiment (Source: http://270c81.medialib.glogster.com/media/96/96afb330969ecc9eba4b9df5f3d79bfe2e406b92fbd04c183befd7b6fa226769/gold-foil-experiment.jpg)
Figure 3: The structure of an atom (Source: https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEi17ih9BlDiJsg6dhWeIj5wppwp2VUwPMp38tc1RBfvomCHRgedUtFPfYQnMQKerHaQvXcXOhvV9szze0FLhgfB-Dys3J9kHdRnbcEm3yEUfXKYbaEz5pMKNIQlLgNZW8LMnzk75EsY1Ag/s1600/Rurtherford.jpg)
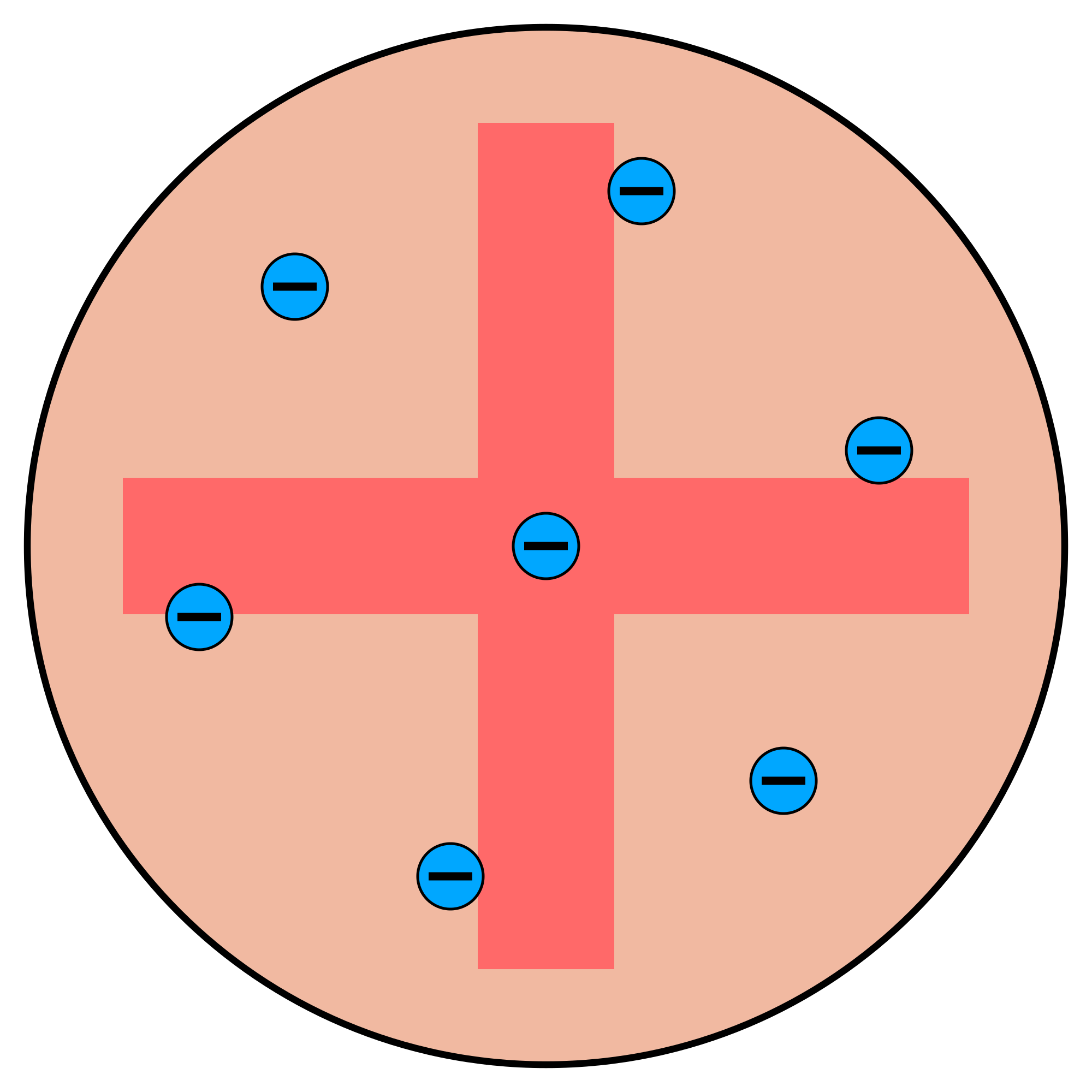
Protons and neutrons were later discovered and which gave a more accurate account of what the structure of an atom looked like (see Figure 4).
However, physicists still were not satisfied with this model which lead to further theories and experiments about what made up an atom. In the 1960s, some ideas that protons and neutrons were made up of smaller particles were proposed and tested leading to the discovery of quarks in CERN in the 1970s. Physicists then came up with the 'Standard Model' suggesting that matter is made up of quarks, leptons and gauge bosons (see Figure 5).
Figure 5: The Standard Model (Source: https://upload.wikimedia.org/wikipedia/commons/0/00/Standard_Model_of_Elementary_Particles.svg
However, this model has been suggested as being 'complicated' and not fundamental enough and theoretical physicists have speculated that all these particles are made up of a fundamental entities called 'strings' and proposed string theory. String theory suggests that all particles are made of bands of different length (related to their energy) and suggest that the way the strings have been tied or if they are open or closed leads to different particles being created (see Figure 6).
Figure 6: How an atom could be made up of 'strings' (Source: http://scienceblogs.com/startswithabang/files/2008/04/3012_elegant_particles.gif)
References
Riley, P. D. (2005). Checkpoint science 3. London: Hodder Education.
Smolin, L. (2006). The trouble with physics: the rise of string theory, the fall of a science, and what comes next. Mariner Books.


.png)


No comments:
Post a Comment